Missed Team ’24? Catch up on announcements here.
×Community resources
Community resources
Community resources
🏆 Affordable, Integrated & Supported: Compliant eQMS on Confluence Cloud
💡 So you had an amazing idea for a revolutionary medical device? Fantastic!
🥳 You put together a group of talented people who are psyched to develop a life-changing device? Congratulations!
💰 You have an idea of how to sell your innovative product once its on the market? Wonderful, you are almost there!!
🤷♀️ Your product meets all the regulatory requirements in markets you are targeting? Wait, what? What requirements?
Let’s start with the fundamentals.
A
The very first thing medical device companies should have in place is the content of the Quality Management System.
A Quality Management System describes the setup of the company, its procedures and practices including the detailed description of your product development.
Medical Device Quality Management System in the EU has to comply with the MDR (more detailed requirements in ISO 13485, IEC 62304, ISO 14971) and in the US, it should be compliant with the FDA 21 CFR 820.
B
A Quality Management System should be electronic to support collaboration and electronic search for quality system documents.
An electronic Quality Management System should have document approval capabilities which means that approvers should be able to electronically sign documents. Electronic Quality/Document Management System should comply with requirements of FDA 21 CFR 11, 21 CFR 820 and ISO 13485.
C
Once you have the content of your Quality Management System and it is all working well on an electronic document management platform, you will need to consider its validation. Software tool validation is yet another requirement for medical device companies - described both in ISO 13485 (clauses 4.1.6 and 7.5.6) as well as in the FDA 21 CFR 820.75 and 21 CFR 11.
With most electronic document management platforms being on Cloud where changes are made often, a manual validation is out of the question. Ideally, validation should be automated for periodic integrity checks of your Quality Management System software providing fully compliant report with documented evidence as a result.
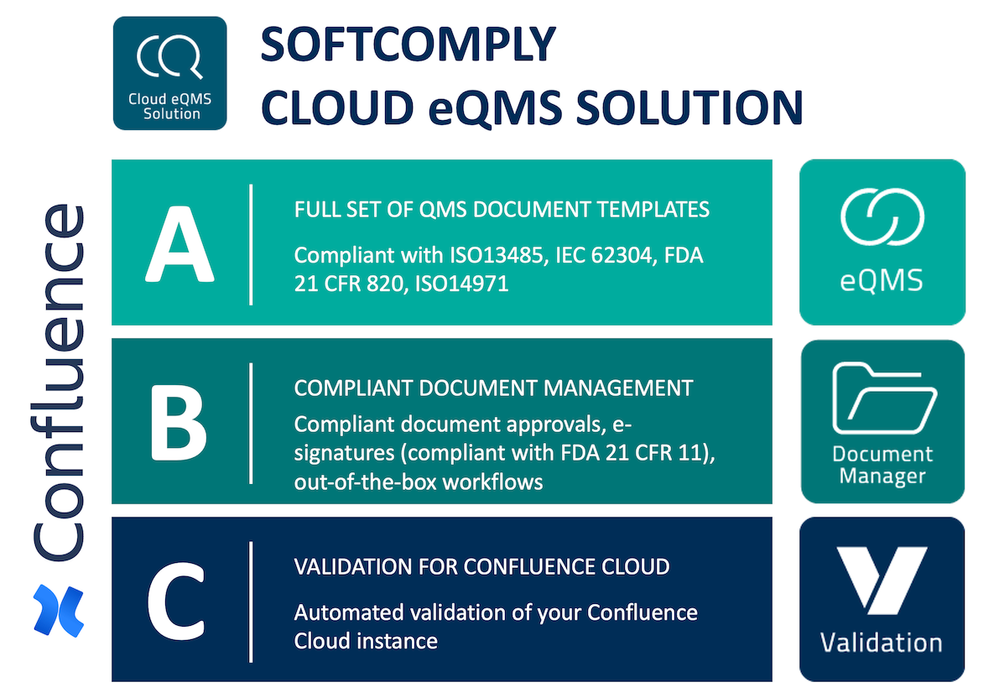
Introducing the SoftComply Cloud eQMS Solution on Confluence
A
SoftComply eQMS Documentation consists of a full set of templates for SOPs and technical documentation with embedded guidance for finalizing it based on your organisation and device. SoftComply eQMS Documentation is based on ISO 13485, IEC 62304, ISO 14971 and FDA 21 CFR 820.
B
SoftComply Document Manager is a Confluence Cloud app that is the central hub for managing your documents: it has several compliant document approval workflows out-of-the-box and FDA 21 CFR 11 compliant e-signatures. Join our live demo sessions to check it out.
C
SoftComply Validation for Confluence Cloud is an app that runs automated validation tests of your Confluence instance once a week after you have set it up. This is a fully supported service. Join our webinar & live demo to see it in action.
Was this helpful?
Thanks!
Marion Lepmets _SoftComply_

About this author
CEO
SoftComply
Munich, Dublin, Tallinn
3 accepted answers
TAGS
Atlassian Community Events
- FAQ
- Community Guidelines
- About
- Privacy policy
- Notice at Collection
- Terms of use
- © 2024 Atlassian





0 comments