Community resources
Community resources
BfB Labs Builds Therapeutic Video Games with Confluence Cloud, Jira and Comalatech
BfB Labs develops therapeutic mobile games to help youth with mental health issues. To manage their development and documentation while meeting medical device regulations like those of the Medicines and Healthcare products Regulatory Agency (MHRA), BfB Labs uses Confluence, Jira and Comala Document Control for Cloud.
Solutions for a Global Crisis in Youth Mental Health
At BfB Labs, the motto is “no young person should go unsupported”. That’s why their games are aimed at the millions of children and youth who struggle to get support for their mental health. The magic is bringing together mobile gaming with proven therapeutic methods like cognitive behavioural therapy. It’s engaging, accessible, and provides evidence-based data for the clinicians. BfB Labs currently has two games and an app for tracking data:
- Champions of Shengha – A fantasy card-battling game for 9-18 year olds that uses a wearable heart rate sensor to teach diaphragmatic breathing.
- Lumi Nova: Tales of Courage – A sci-fi game for 7-12 year olds that uses exposure therapy to build life-long skills for managing worries and anxiety.
- VitaMind Hub – An app that allows clinicians, teachers and other specialists to track progress in real-time, and download reports.
Building Documentation that Meets Medical Device Regulations
All three apps are classified as medical devices and need to meet the appropriate regulations. In the UK that means the MHRA, which also automatically qualifies them for FDA standards. “Getting medical devices approved is a mammoth task,” says Manjul Rathee, BfB Labs’ CEO. “It takes a lot of effort and rigour. And a big part of that is documentation and change management.”
BfB Labs started with Google Docs for their documentation, but had reservations because of the platform’s data and privacy issues. With a lean team, they needed a system that would be efficient, secure and allow them to work together remotely. Atlassian tools fit the bill, and the ability to customize the products with add-ons meant they could create a system to match the needs of compliance regulators. Today, they use Jira for product development and project management, while Confluence Cloud serves as their documentation workspace.
As a collaboration tool, Confluence allows their remote team to come together and work on documents at the same time, without having to pass files back and forth. The Confluence instance is divided primarily into technical specifications and data protection specifications. MHRA regulations are at the heart of their set up, with each page using an appropriate template created by their medical device experts. Confluence’s native ability to track document versions is also an essential tool for meeting compliance regulations.
Setting up Workflows with Digital Signs-offs
The next component they required was an approval process. A quick Google search brought them to Comala Document Control, an app which offers the ability to create workflows for Confluence documents with digital signatures. “We had clear need for something that would allow us to achieve that document sign-off, and in a way that makes it easy for teammates to see which stage the document is at,” Manjul says. “With Comala Document Control there’s no need to extract documents out of Confluence, sign it, and put it back in.”
BfB Labs uses the Basic Approval Workflow, one of three built-in workflows that comes with Comala Document Control. Organizations can also design their own custom workflows, but for many like BfB Labs, these three templates will be enough to achieve their goals, including many compliance regulations.
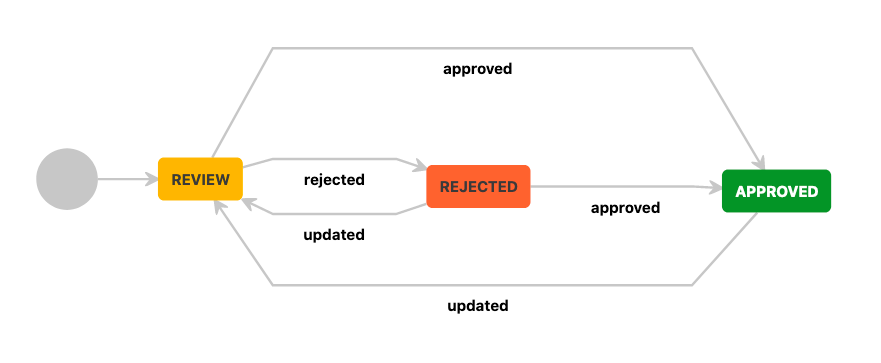
The Basic Approval Workflow begins with the document in a Review state, which must be approved or rejected by one or more assigned reviewers. If approved, the page moves to the Approval state. If the document is rejected, it will transition to the Rejected state, where it can be edited and updated to return it to Review. Any later changes to the document after it has been approved will also revert the document to the Review state. With this workflow, the team at BfB Labs is able to collaborate together on documents, receive approvals from subject experts, and finally from the CEO herself.
To add sign-off functionality, an important requirement of compliance, BfB Labs uses the app’s built-in e-signatures. When an approval is given, reviewers must enter in their username and a one-time password. The feature works with popular two-factor authentication apps like Authy, Google Authenticator and 1Password to generate time-based tokens, and these signatures are recorded along with all approvals, state changes and versioning information in a report.
A Successful App Launch
With the help of Jira, Confluence and Comala Document Control, BfB Labs was able to meet MHRA regulations. “Comala Document Control has been very functional for us,” Manjul says. “We advocate it to other people in our position who are trying to achieve a medical device regulation and have a heap of documentation!”
The apps launched on September 2nd, 2020 and have already seen great success. Champions of Shengha won the Best Mental Health Technology Solution award from Health Tech Digital Awards, and BfB Labs has received coverage from publications like the BBC, The Times, and RC Psych. A live community of young people are testing the apps and providing regular feedback, while almost 1000 licenses have already been sold to various interventions.
Interested in learning more about BfB Lab’s games? Visit their website and get in touch by email. You can learn more about Comala Document Control and get a free trial from the Atlassian Marketplace.
Was this helpful?
Thanks!
Lua_Boschman__Comalatech_
TAGS
Atlassian Community Events
- FAQ
- Community Guidelines
- About
- Privacy policy
- Notice at Collection
- Terms of use
- © 2024 Atlassian






0 comments