Community resources
Community resources
Community resources
A Customer Story: Agile eQMS on Confluence Cloud

A Customer Story on building & maintaining a compliant eQMS at agile speed on Atlassian Confluence and Jira
Orthogonal, a Chicago based company, builds SaMD and connected device systems by combining agile methods with regulatory compliance requirements. Over the course of the last 10 years they have been working with companies of various sizes – from device manufacturing start-ups to pharmaceutical giants that build, evolve and scale the regulated, software-enabled medical devices.
Maintaining their eQMS to fit agile development has always been a challenge and the main reason why Orthogonal has been looking for a solution that best meets these controversial requirements.
Working on a SaMD you need to adopt an Agile eQMS. Manage compliance in the same environment your developers use and let them develop. It creates less friction, eliminates the duplication of work and speeds up your progress, resulting in faster market access.
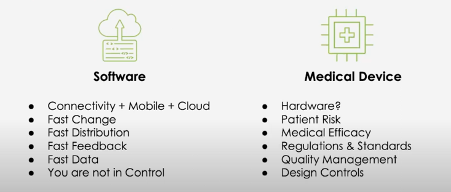
THE CONTROVERSY
When we think about software, the central keyword is: Speed
When we think about Medical Devices, the most important keyword is: Safety
THE SOLUTION – an Agile eQMS
Prior to the new ways of working, Orthogonal had a typical document-centric standalone system for Requirements, Specifications, Risk Management, Verification, Traceability. At the same time, their Developers, Product Managers and QA were working in Atlassian Jira, Confluence and GitHub. There was not much synergy between these processes – people were not looking at/working on the same things in the same environments. This resulted in having documentation for documentation’s sake, wasted effort, longer timelines. All of them knew that there must be a better way of doing this.
The key is to make non-development activities more agile.
The aim was to get the whole team working within one system, where modular design and agile practices could be extended to design control activities. All this was to be achieved with minimal developer disruption: minimal developer disruption: let the developers develop and get their input using the same tools they had been using. Integrated, iterative work on Requirements, Architecture, Design, Risk Management, V&V and Traceability needs to be visible to everyone.
Documentation should be generated automatically as the output of work being done, rather than an extra effort of manually producing documentation. In a nutshell, Orthogonal was aiming for: increased quality, reduced cost and reduced effort spent on design controls.
SETTING UP eQMS ON ATLASSIAN CONFLUENCE AND JIRA
There are a number of add-ons in Jira and Confluence that can be used to support your electronic document management.
Orthogonal collaborated with SoftComply who helped kick-start their documentation management in Confluence by applying compliant workflows and configuring all the relevant apps they were using. “That saved us at least 3 months of time from (con)figuring everything out ourselves”, said Orthogonal’s Chief Solution Officer.
Orthogonal chose the following SoftComply add-ons on Atlassian platform:
- SoftComply eQMS
- SoftComply Static Snapshots
- SoftComply Change History
- SoftComply Risk Manager in Jira
- SoftComply Risk Manager for Confluence
“Having the support from vendors like SoftComply and using tools like the SoftComply Risk Manager really facilitate the overall experience of development management and document”.
Please, see the webinar recording from Orthogonal to see how they put these apps into use, and more.
Full article by Orthogonal can be found here.