Community resources
Community resources
Neurotrack Builds Compliant QMS for their Cognitive Health App with Confluence and Comalatech
Neurotrack develops digital solutions that help people assess, monitor and improve their cognitive health. To meet regulatory standards like HIPAA and control their documentation, they use Confluence Cloud with SoftComply, Comala Document Management and Comala Publishing.
A Clinically-Validated Cognitive Health App
Neurotrack’s team of neuroscientists and engineers have developed an app that provides cognitive testing for conditions like Alzheimer’s disease and dementia, allowing health professionals to perform these tests remotely. To date, over 25,000 people have used the app, and Neurotrack is in the process of rolling out a coaching program to help individuals further strengthen their cognitive health.
Today the solution is available as a mobile app in Japan, and can be used in the US through a web app.
An Online Work Environment for a Remote-first Company
While supplying remote testing solutions, Neurotrack has become a remote-first company itself, with teams located in Japan, the USA, and Canada. Even before they went fully remote, they used Atlassian products like Confluence Cloud, a team collaboration platform, as an important part of their workflow.
“It’s very important for me as VP, that we find ways to communicate simultaneously and asynchronously,” says Jeff Magnussen, Neurotrack’s VP of Engineering. “Confluence has been an important part of moving our documentation into an online environment where that can happen.”
Neurotrack has around 50 Confluence users, and traditionally their instance has been broken up into functional silos like Engineering, Clinical, and Compliance. Today they are evolving to collect their most important documentation into a central handbook space, to minimize siloing. As they adopt this handbook-first approach, they saw a need to add ownership and approvals to these essential documents.
“Confluence allows us to create a permanent source of truth, that bridges both the operational day-to-day needs, and the needs of being asynchronous. That source of truth is also necessary for meeting compliance standards like FDA,” explains Jeff. To provide that extra layer of control, they turned to Confluence vendors, SoftComply and Comalatech.
Creating a Compliant QMS in Confluence Cloud
While there are many platforms for developing quality management systems, Neurotrack wanted something that could live in their existing environment. “I’ve worked with a variety of QMS software. I wanted one that sat inside of Confluence,” explains Katie Wallace, Senior Compliance Engineer.
They turned to SoftComply, providers of compliance solutions for Atlassian tools. Along with furnishing a complete QMS set-up in Confluence with compliant document templates, SoftComply also suggests Comala Document Management and Comala Publishing as part of their solution. Comala Document Management is an app that lets you create approval process workflows in Confluence, while Comala Publishing allows you to easily copy content from a draft to a published space. In particular, Comala Document Management provides some essential tools for meeting many compliance standards, like electronic signatures, approvals and audit-ready reporting. With SoftComply and Comalatech apps, Neurotrack was able to build a compliant documentation set up that worked with their existing system. “This was definitely the simplest, lightest option for us,” Katie says.
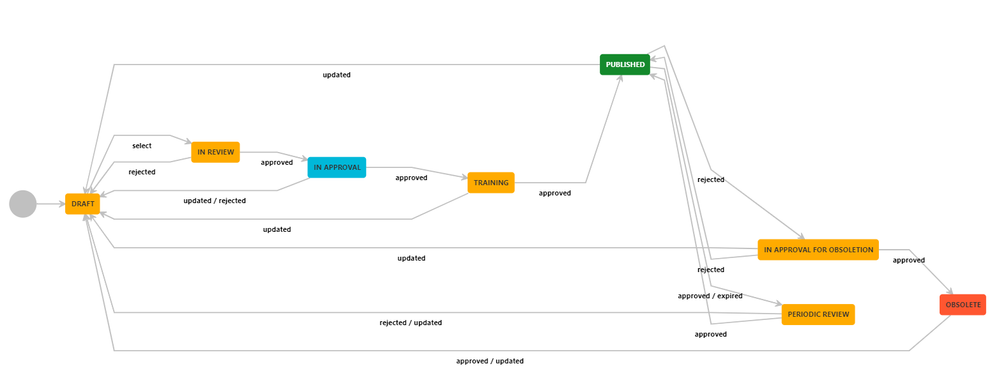
Documents in their QMS are created in an “eQMS Draft” space where they go through an approval process, before the finalized versions are published to the “QMS Published” state and made available to other teams in the company. This process is performed through a Comala Document Management workflow:
The document starts in a DRAFT state where it is written and edited. Once it’s ready, it can be submitted to the In REVIEW state. At this point, it is reviewed and approved or rejected by the VP of Engineering. This approval requires an electronic signature, using the VP’s username and a two-factor authentication. Jeff uses 1Password to create his token, one of the many authentication apps that integrates with Comala Document Management.
If the document is rejected it reverts to the DRAFT state; if approved it goes to the IN APPROVAL state, where it is reviewed by Katie, as the compliance expert. She also enters an e-signature, this time using her app of choice, Google Authenticator, to generate a token. From there, the document passes into a TRAINING state, so select team members can be trained on the new documentation. Once it has been approved, the document goes to the PUBLISHED state, and is automatically published to the “QMS Published” space using the integration with Comala Publishing. Here, the fully-approved document is made available to the entire Neurotrack team.
After a year, the workflow will automatically notify them to perform a review, fulfilling an important requirement for compliance. At this point, they can either obsolete the document, or re-approve. If the document is updated at any point, it will revert to the DRAFT state for another round of reviews.
With this set up, team members can always read the latest finalized version in the “QMS Published” space, while select users work on the newest draft in private. Comala Document Management also has built-in reporting, providing a record of page changes and approvals, which is essential for audits.
This QMS workflow has allowed Neurotrack to control HIPAA Privacy and Security Policy documentation, and it is an important step in their current application for FDA compliance. While the workflow was designed by SoftComply, Neurotrack is able to customize it and develop other workflows using Comala Document Management, like the one they are building for their Confluence handbook.
With a successful track record delivering remote cognitive assessments in Japan, Neurotrack is poised to expand into new markets and deliver more solutions with monitoring and coaching capabilities. Using SoftComply, Comala Document Management and Comala Publishing they have built a lightweight QMS that meets compliance needs while working in their existing Confluence Cloud environment.
“We need powerful document management capabilities. Finding a tool that met us where we are, and where our users work, is very valuable,” says Jeff. “Now we have sophisticated capabilities, without having to adopt new tools.”
To learn more about Neurotrack and their apps, visit neurotrack.com. You can learn more about Comala Document Management and Comala Publishing and try both apps for free for 30 days on the Atlassian Marketplace.
Was this helpful?
Thanks!
Lua_Boschman__Comalatech_
TAGS
Atlassian Community Events
- FAQ
- Community Guidelines
- About
- Privacy policy
- Notice at Collection
- Terms of use
- © 2024 Atlassian





2 comments